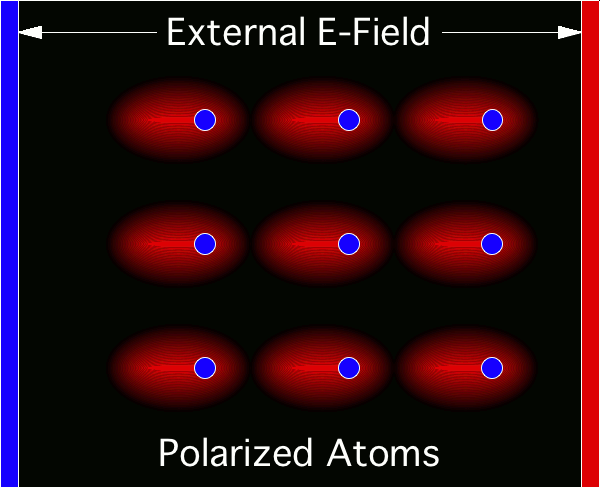
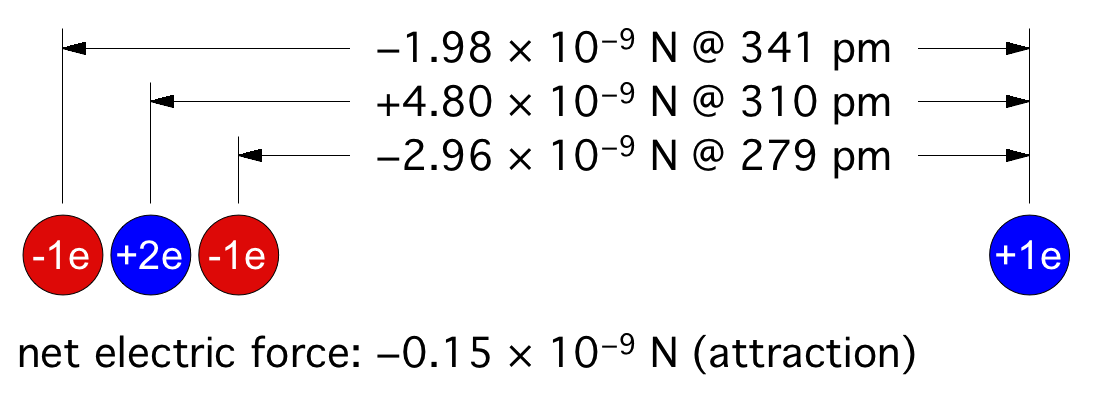© Charles ChandlerThere is an organizing principle in physics that operates across a broad range of scales, from the force that binds atoms together into molecules, to the force that organizes stars into galaxies — it's the electric force.Figure 1. Electric forces between a neutrally charged helium atom at left, with 2 electrons as point sources at the K shell radius of 31 pm, and a positive test charge at right, arbitrarily 310 pm from from the helium nucleus, showing a slight net attraction.1 At the same distances, gravity is just 7.77 × 10−45 N, so it isn't a factor.Richard Feynman was the first to realize that there is a hidden significance to the electric force's inverse square law that is important in bringing atoms together to make molecules.2:ch2:pg2,3,4,5,6,7 He said that it requires ionization, because a positive ion is attracted to a neutrally charged atom. The reason is that the negative charges in the neutral atom's electron cloud get nearer to the distant positive ion. Then if we just look at the effects of the inverse square law, we realize that the near-side electrons exert a greater attraction per elementary charge on the positive ion than the repulsion from the atomic nucleus, and the attraction to the far-side electrons isn't that much less. If we add up all of these +/− forces, we find that the neutral atom shows a slight net negative charge at a distance. (See Figure 1.)This means that any nearby positive ion will be pulled toward the neutral atom, despite the net charge of the whole assembly being positive (ergo the paradoxical "like-likes-like" effect). We should also note that the attraction gets stronger as the positive ion gets nearer to the neutral atom, since the relative proximity of the positive ion to the near-side electrons gets more dramatic. It's also possible (depending on which atomic model is used) that the electrons favor that near side, being attracted to the positive ion. If there is even more negative charge on the near side of the neutral atom, the positive ion will experience an even greater attraction. So the positive ion is pulled toward the neutral atom, to the point of sharing electrons with it, thereby forming a molecule.The corollary is that if both atoms are neutrally charged, they will both be showing a net negative charge at a distance, and they will repel each other. This is why the elements with fully populated outer electron shells (i.e., the noble gases) are the least likely to form molecules — electrostatic repulsion within the shells calls for an equal distribution of electrons, and if the shell is fully populated, there is nowhere for electrons to go if exposed to a negative test charge at a distance, so they stay where they were, and push back. This is also why the elements with the lowest ionization potentials are the best at forming molecules — once one of the atoms gets ionized, the net electric force goes from repulsive to attractive.*14532Figure 2. The charges within atoms get polarized in an external electric field, producing an attraction between them.A related phenomenon is the molecule-building facilitated by an external electric field, which chases electrons to the far sides of their atoms, making electric dipoles out of the atoms. With the negative side of one atom facing the positive side of the other, there is an electrostatic attraction between the atoms. (See Figure 2.) This can turn a gas into a liquid, a liquid into a liquid crystal,3,4,5,6 or a gas into a polymerized solid.This same principle also acts on an astronomical scale. In the beginning, nothing existed except huge clouds of weakly ionized gas & dust, known as dusty plasmas. Somehow, these extremely diffuse clouds collapsed into very dense stars, planets, & moons, but Newtonian physics can't explain how this ever could have happened. Gravity will pull the matter inward, but if it succeeds in compressing the gas, the hydrostatic pressure inside the gas will push back out. As the compression continues, the outward force of internal pressure will increase faster than the inward force of gravity, meaning that eventually, the pressure will be as strong as gravity. Once the equilibrium has been achieved, further compression can't happen, because there isn't any net force. For example, the Earth's atmosphere is far cooler than a dusty plasma if compressed down to the same density,8 and our atmosphere is under the Earth's gravity, which is a lot stronger than the gravity inside a dusty plasma with nothing else around, and yet the air doesn't collapse under its own weight, because the hydrostatic pressure won't let it.Such being the case, it's no surprise that modern telescopes have detected primordial plasmas that refuse to collapse, some of which seeming to have been around since the beginning of time. If they were going to collapse under their own weight, they already would have. If they haven't already, it's because gravity alone isn't enough — there has to be another force.Studying the conditions in which a dusty plasma collapses reveals the nature of that force. The dusty plasma has to be hit by the ejecta from a supernova, or it has to collide with another dusty plasma. What's the significance? It isn't that the collapse has been facilitated by a stronger gravitational attraction in denser matter, because there will also be an increase in hydrostatic pressure that will more than offset the gravity. Furthermore, the combined velocities in such collisions are typically above 20 km/s, and the thermalization of hypersonic collisions should greatly increase the hydrostatic pressure, causing the expansion (or even the explosion) of the mixture.
1. Chandler, C. (2022): Molecule Building Forces. QDL, 17079 ⇧
2. Feynman, R.; Leighton, R.; Sands, M. (1970): The Feynman Lectures on Physics. Reading, MA, USA: Addison-Wesley ⇧
3. Nagornyak, E.; Pollack, G. H. (2005): Connecting filament mechanics in the relaxed sarcomere. Journal of Muscle Research and Cell Motility, 26 (6-8): 303-306 ⇧ ⇧
4. Pollack, G. H.; Figueroa, X.; Zhao, Q. (2009): Molecules, Water, and Radiant Energy: New Clues for the Origin of Life. International Journal of Molecular Sciences, 10 (4): 1419 ⇧ ⇧
5. Zhao, Q.; Coult, J.; Pollack, G. H. (2010): Long-range attraction in aqueous colloidal suspensions. Proceedings of the Society of Photo-Optical Instrumentation, 7376: 73761C1-73761C13 ⇧ ⇧
6. Pollack, G. (2013): The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor. Seattle: Ebner and Sons Publishers ⇧ ⇧
7. dos Santos, A. P.; Levin, Y. (2019): Like-Charge Attraction between Metal Nanoparticles in a 1:1 Electrolyte Solution. Physical Review Letters, 122 (24): 248005 ⇧
8. Chandler, C. (2019): Dusty Plasma to STP Density. QDL, 14095 ⇧
9. Chandler, C. (2022): Accretion. QDL, 12692 ⇧ ⇧
10. Mott-Smith, H. M. (1971): History of "Plasmas" Nature, 233 (5316): 219 ⇧
11. Delgado-Serrano, R. et al. (2010): How was the Hubble sequence 6 Gyr ago? Astronomy and Astrophysics, 509 (A78): 1-11 ⇧
12. Mo, H.; van den Bosch, F.; White, S. (2010): Galaxy Formation and Evolution. ⇧
13. May, H. D. (2008): A Pervasive Electric Field in the Heliosphere. IEEE Transactions on Plasma Science, 36 (5): 2876-2879 ⇧
14. May, H. D. (2010): A Pervasive Electric Field in the Heliosphere (Part II). viXra, Astrophysics: 1005.0090 ⇧
15. Kaal, J. E.; Sorensen, J. A.; Otte, A.; Emming, J. G. (2021): The Structured Atom Model. ⇧