Electron Structure
http://symmetrymath.com/sitebuilder/images/electron2-350x21~

N: North; h: ?; Me: Electron Magnetic Field; re: Electron Radius; pe: Torus Cross-section Radius; S: South
- The Electron has a slinky Torus shape
- The slinky is the path of a high-speed particle
- The particle makes 6 helical turns per revolution around the center
- Protons, Neutrons and Photons have similar Torus structure
- The Electron's Electric Field Is Apple-shaped with vertical axis
- Its Magnetic Field Has vertically rotated Figure-8 Shape
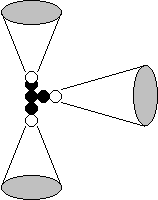
Hydrogen Structure
http://i573.photobucket.com/albums/ss180/Lkindr/TB/HAtom.jpg

Me: Electron Magnetic Field; e: Electron; h: height?; P: Proton; Mp: Proton Magnetic Field
Proton Diameter: 2.6x10^-15m
Electron Diameter: 4.8x10^-12m = 1846 times the Proton Diameter
Proton-Electron Distance: 10^-9m = 385,000 times the Proton Diameter
- Hydrogen is an Electron-Proton Rod
- The Electron does not orbit the Proton; It hovers above the Proton
- Opposite Electric Fields Draw the Electron & Proton Together
- Same Magnetic Poles Hold Them Apart
Nuclear Structure
- Each nucleus has 3 perpendicular axes, x, y & z
- Neutrons occupy the centers of each axis
- Protons occupy the outer portions, separated by central Neutrons
http://www.sciteclibrary.ru/ris-stat/3418/image10877.gif

The Graphite & Diamond Atoms in the image are Carbon Atoms.
Black & Gray Balls are Neutrons; Plain White Balls are Protons; White Balls marked "e" are Electrons.
http://guns.connect.fi/innoplaza/energy/story/Kanarev/coldf~
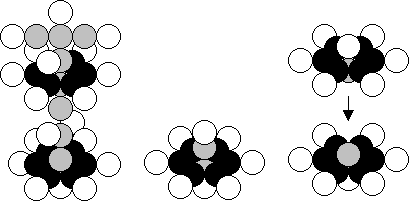
a) K (19,20) ------------------ b) O (8,8) ------------------ c) Si (14,14)
Fig. 3. Diagrams of the atomic nuclei of: a) potassium, b) oxygen, c) silicon
* Here are more Atomic Structures.
[url]H-Be: http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/b~
[url]Na-Sc: http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/b~
[url]Ti-Cu: http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/b~
* This section shows more of the exact structure with the electrons, which make atoms appear cone-shaped. Below is Lithium.
http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/b~
http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/b~