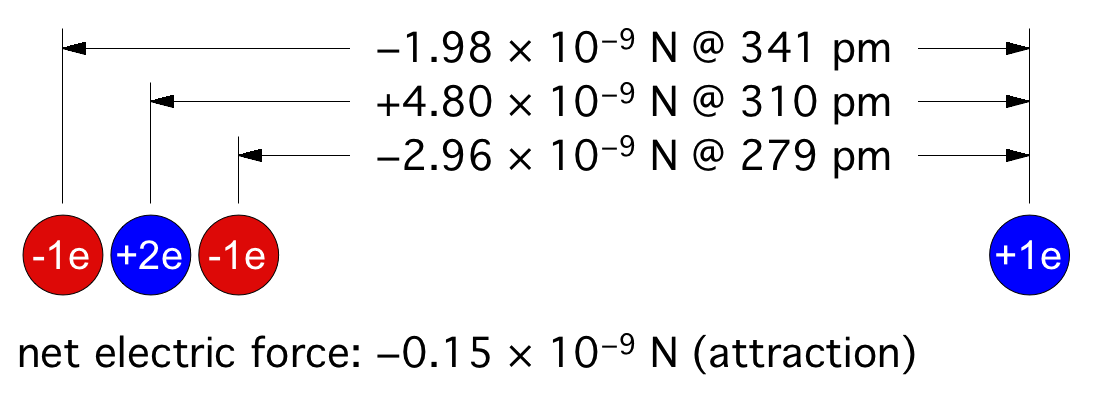© Charles ChandlerFigure 1. Electric forces between a neutrally charged helium atom at left, with 2 electrons as point sources at the K shell radius of 31 pm, and a positive test charge at right, arbitrarily 310 pm from from the helium nucleus, showing a slight net attraction.1 At the same distances, gravity is just 7.77 × 10−45 N, so it isn't a factor.Richard Feynman was the first to realize that there is a hidden significance to the electric force's inverse square law that is important in bringing atoms together to make molecules.2:ch2:pg2,3,4,5,6,7 He said that it requires ionization, because a positive ion is attracted to a neutrally charged atom. The reason is that the negative charges in the neutral atom's electron cloud get nearer to the distant positive ion. Then if we just look at the effects of the inverse square law, we realize that the near-side electrons exert a greater attraction per elementary charge on the positive ion than the repulsion from the atomic nucleus, and the attraction to the far-side electrons isn't that much less. If we add up all of these +/− forces, we find that the neutral atom shows a slight net negative charge at a distance. (See Figure 1.)This means that any nearby positive ion will be pulled toward the neutral atom, despite the net charge of the whole assembly being positive (ergo the paradoxical "like-likes-like" effect). We should also note that the attraction gets stronger as the positive ion gets nearer to the neutral atom, since the relative proximity of the positive ion to the near-side electrons gets more dramatic. It's also possible (depending on which atomic model is used) that the electrons favor that near side, being attracted to the positive ion. If there is even more negative charge on the near side of the neutral atom, the positive ion will experience an even greater attraction. So the positive ion is pulled toward the neutral atom, to the point of sharing electrons with it, thereby forming a molecule.The corollary is that if both atoms are neutrally charged, they will both be showing a net negative charge at a distance, and they will repel each other. This is why the elements with fully populated outer electron shells (i.e., the noble gases) are the least likely to form molecules — electrostatic repulsion within the shells calls for an equal distribution of electrons, and if the shell is fully populated, there is nowhere for electrons to go if exposed to a negative test charge at a distance, so they stay where they were, and push back. This is also why the elements with the lowest ionization potentials are the best at forming molecules — once one of the atoms gets ionized, the net electric force goes from repulsive to attractive.*14532
1. Chandler, C. (2019): Molecule Building Forces. QDL, 17079 ⇧
2. Feynman, R.; Leighton, R.; Sands, M. (1970): The Feynman Lectures on Physics. Reading, MA, USA: Addison-Wesley ⇧
3. Nagornyak, E.; Pollack, G. H. (2005): Connecting filament mechanics in the relaxed sarcomere. Journal of Muscle Research and Cell Motility, 26 (6-8): 303-306 ⇧
4. Pollack, G. H.; Figueroa, X.; Zhao, Q. (2009): Molecules, Water, and Radiant Energy: New Clues for the Origin of Life. International Journal of Molecular Sciences, 10 (4): 1419 ⇧
5. Zhao, Q.; Coult, J.; Pollack, G. H. (2010): Long-range attraction in aqueous colloidal suspensions. Proceedings of the Society of Photo-Optical Instrumentation, 7376: 73761C1-73761C13 ⇧
6. Pollack, G. (2013): The Fourth Phase of Water: Beyond Solid, Liquid, and Vapor. Seattle: Ebner and Sons Publishers ⇧
7. dos Santos, A. P.; Levin, Y. (2019): Like-Charge Attraction between Metal Nanoparticles in a 1:1 Electrolyte Solution. Physical Review Letters, 122 (24): 248005 ⇧